Product Details
KBroVet®-CA1 is a safe,
reliable treatment for control of seizures associated with IE
KBroVet-CA1 is the first drug conditionally approved by the FDA for the control of seizures associated with idiopathic epilepsy in dogs. It was developed to provide a consistent and reliable source of potassium bromide, which may help control the seizures associated with IE in canine patients.
The delicious tablet is given once daily, so you don’t have to manage a complex schedule of multiple doses each day. And while it doesn’t make your dog’s seizures go away completely, it can bring some stability to the storm and allow you to get back to a more comfortable daily routine.


The KBroVet®-CA1 Advantage
Conditional FDA approval
As a step toward full approval, the FDA has determined that this product has been demonstrated to be safe with a reasonable expectation of effectiveness.
21 day half-life in bloodstream
If you miss a dose, drug concentration fluctuation is unlikely to occur, which minimizes the risk of a seizure. (And gives you peace of mind.)
1x per day
Easy on dogs’ livers
Delicious flavor
French vanilla-flavored tablets formulated specifically for dogs, making treatment easy.
Convenient packaging
60- and 180-count bottles available for convenient storage and dosing.
What does “conditional approval” mean?
KBroVet-CA1 has received conditional approval from the FDA, so you can trust that it’s a safe and reliable choice for your dog.
Conditional approval means that a product has been proven to be safe and has a reasonable expectation of effectiveness when used according to the label. This provides a faster timeline for products to be legally available, giving veterinarians more treatment options for their patients. The sponsor will continue to collect evidence of effectiveness needed for the product to receive full approval.
It is a violation of Federal Law to use this product other than as directed in the labeling. Learn more about conditional approval at FDA.gov.
Reasonable expectation of effectiveness study of KBroVet®-CA1
Determination of Success
Determination of Success
- The sponsor evaluated the medical records of 51 client-owned dogs that were previously treated with KBr to control their idiopathic epilepsy.
- Effectiveness was evaluated by comparing the 30-day period before initial treatment with KBr and the 30-day period of steady state KBr dosing using these criteria:
- Seizure counts – decrease of ≥50%
- Seizure event days per month – decrease of ≥50%
- Seizure severity scores – decrease or no change
- Overall reasonable expectation of effectiveness was achieved if >50% of all cases achieved a “success” score for all three variables.
Study Results
As the sole antiepileptic, KBr achieved 67% overall treatment success in all three categories:
Seizure Counts
70% (19/27) were defined as treatment successes
Seizure Event Days Per Month
67% (18/27) were defined as successes by either decreasing or showing no change
Seizure Severity
93% (25/27) were defined as successes by either decreasing or showing no change
Potassium bromide is the active ingredient in KBroVet®-CA1
Potassium bromide is a well-known choice for long-term control of seizures associated with idiopathic epilepsy in dogs.2 The mean elimination half-life of potassium bromide is 21 days.1
The science: Potassium bromide is thought to exert its antiepileptic activity by passing through the neuronal chloride ion channels, thereby hyperpolarizing neuronal membranes, raising the seizure threshold, and stabilizing neurons against excitatory input from epileptic foci.3
Your daily reality: The active ingredient calms the neurons in your dog’s brain cells, which means your dog experiences fewer seizures.
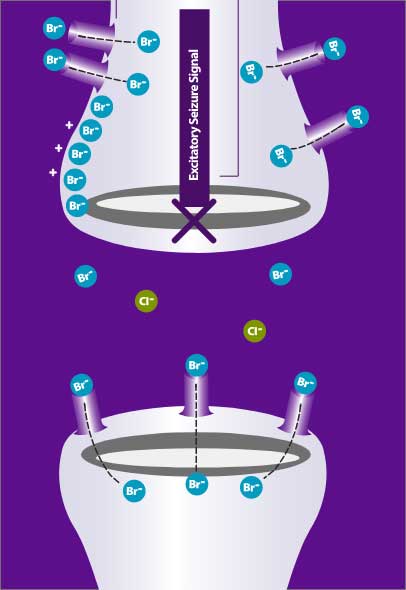
Animation video explaining potassium bromide mode of action
Dosage & Administration

Dosage & Administration
Your veterinarian will determine the best daily dose for your dog. The total recommended daily dosage range for oral administration is 25–68 mg/kg (11–31 mg/lb) of body weight. The dosage of KBroVet-CA1 should be adjusted based on monitoring of clinical response of the individual patient. KBroVet-CA1 may be dosed with or without food.4 Use of an initial loading dosage regimen may be considered on an individual patient basis, balancing the time required to achieve a therapeutic response while minimizing side effects.
Precautions
Dogs receiving KBroVet-CA1 should be carefully monitored when changing diets, administering chloride-containing intravenous fluids, and administering concurrent medications. Careful monitoring is important in dogs that have a condition that may cause difficulty maintaining electrolyte balance.
- Animals with decreased renal function may be predisposed to bromide toxicosis.
- Some dogs may experience epileptic episodes that are unresponsive or refractory to KBr monotherapy and KBr alone may not be adequate for control of seizures for every dog with idiopathic epilepsy.
- The safe use of KBroVet-CA1 has not been evaluated in dogs that are intended for breeding, or that are pregnant or lactating. The safe use of KBr in neonates and young animals has not been established.
- Reproductive effects of KBr have been reported in other species.
- In dogs, ataxia, diarrhea, hematochezia, excessive salivation, shivering, skin lesions, stupor progressing to coma, and death have been reported.
Talk to your veterinarian about KBroVet®-CA1
Be prepared to discuss treatment options and ask your veterinarian if KBroVet-CA1 might be the right choice for your dog. KBroVet-CA1 is only available by prescription from a licensed veterinarian.
1. Boothe, D, Dewey C, Carpenter D. Comparison of phenobarbital with bromide as a first-choice antiepileptic drug for treatment of epilepsy in dogs. JAVMA. 2012; Vol 240, No 9. 1073-1083.
2. Baird-Heinz HE, Van Schoick ANL, Pelsor FR, et al. A systematic review of the safety of potassium bromide in dogs. JAVMA. 2012;240(6):705-15.
3. Nettifee JA, Munana KR, Griffith EH. Evaluation of the impacts of epilepsy in dogs on their caregivers. J Am Anim Hosp Assoc. 2017;53(3):143-149.
4. Podell M, Fenner WR. Bromide therapy in refractory canine epilepsy. J Vet Intern Med. 1993; 7(5): 318-27.
